Atomic Structure and Isotopes
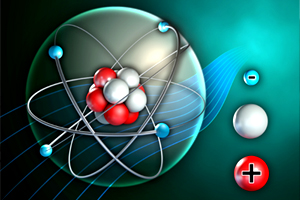
Atoms are the smallest particles of elements that retain the characteristics of those elements. In this course, students will learn how to describe the electrical charge and location of the subatomic particles in an atom, identify isotopes, and distinguish between an atomic mass and an atomic number of an atom.
Prerequisite: Basic knowledge of the periodic table
undefined
-
1Lesson # 1: introduction, overview of the course, and evaluation of students' chemistry background.
-
2Lesson # 2: Nucleus, protons, neutrons, and electrons. Dynamic modules exercises. Practice problems.
-
3Lesson # 3: Identifying Isotopes. Dynamic modules exercises. Practice problems.
-
4Lesson # 4: Summery of course content. Problem solving session.
Be the first to add a review.
Please, login to leave a review


